Worldwide attention for IBD drug candidate developed in Kiel
An important milestone for the drug candidate "Olamkicept" developed by PMI Cluster members on the way to approval: The high-ranking Journal of the American Medical Association (JAMA) has published a Phase II study confirming the efficacy and tolerability of the novel drug in patients with ulcerative colitis.
"Congratulations on this success," says Prof. Dr. Dr. h.c. mult. Jens Scholz, CEO of the University Medical Center Schleswig-Holstein (UKSH), "because it impressively demonstrates that we in Schleswig-Holstein are succeeding in bringing the results of cutting-edge medical research directly to the patient's bedside as innovative therapy." Prof. Dr. Joachim Thiery, Dean of the Faculty of Medicine at Kiel University (CAU), added: "The successful Phase II study in JAMA now gives new hope to many patients with inflammatory diseases. The path from the discovery of a new biochemical principle to clinical application follows our guiding principle of sustainability in medical research. This success is a milestone in precision medicine." Prof. Dr. Stefan Schreiber, Director of the Department of Internal Medicine I at the UKSH, Campus Kiel, and speaker of the Cluster of Excellence "Precision Medicine in Chronic Inflammation" (PMI) says: "The fact that the functioning of our therapy mechanism is now attracting international attention is very good news for patients, as approval is within reach."
Prof. Schreiber has significantly advanced the development of the active substance with his teams. The inventor of the molecule, the Kiel biochemist Prof. em. Dr. Stefan Rose-John, is proud of the success that the decades of preliminary work could actually be transferred to humans. "This is by no means a matter of course. Many molecules work in animal models, and only rarely can the successes achieved there be transferred to humans. With the biochemical principle we have found, we have opened the door to a completely new inflammatory medicine," says Professor Rose-John, who is also a member of the Cluster of Excellence PMI.
Ulcerative colitis is a chronic inflammation of the large intestine that usually occurs in episodes and is characterized by recurring diarrhea, intestinal bleeding and colic. It affects more than 150,000 people in Germany. The inflammation is caused by a misdirected immune reaction in which the immune system mistakenly attacks cells in the colon. Due to the different individual manifestations of the disease, currently available medications only work for a small number of sufferers and often cause side effects, meaning that new therapeutic approaches are urgently needed to help sufferers for whom previous therapeutic approaches have been unsuccessful.
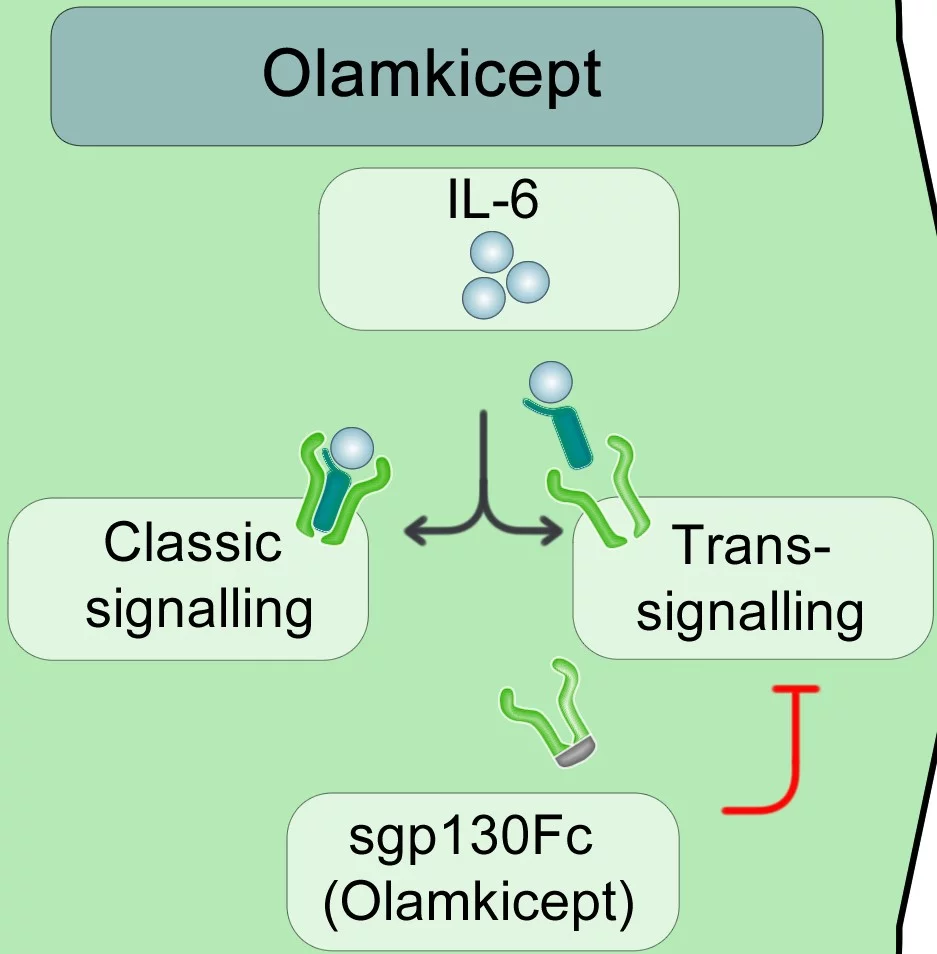
Olamkicept is based on a novel mode of action that none of the approved drugs for ulcerative colitis have used to date. During inflammation, as occurs in ulcerative colitis, the body releases an increased amount of the signaling molecule interleukin-6 (IL-6). However, if all the effects of IL-6 are blocked - as with previous medications - this is very successful in reducing inflammation, but at the same time the immune system can be suppressed to such an extent by blocking IL-6 that the body becomes significantly more susceptible to infections.
The special thing about Olamkicept is that it specifically blocks the so-called IL-6 trans-signaling pathway. This signaling pathway and the blocking protein sgp130Fc were discovered by the Kiel biochemist Prof. Dr. Stefan Rose-John, who has done pioneering work in their research. Together with researchers from the Cluster of Excellence "Precision Medicine in Chronic Inflammation", he further developed the sgp130Fc protein, which was finally tested in clinical trials as the drug candidate Olamkicept. A first study with 16 patients at the UKSH, Campus Kiel, was able to demonstrate the basic functioning of the therapeutic mechanism of Olamkicept in 2021.
The study, which has now been published in the renowned journal JAMA, once again demonstrated the efficacy and tolerability of olamkicept in patients with ulcerative colitis from various Asian countries. In the Phase II study conducted by I-Mab Biopharma, 91 patients with moderate or severe ulcerative colitis were given either a placebo, 300 mg olamkicept per day or 600 mg olamkicept per day for twelve weeks. The study was double-blind, i.e. neither the patients nor the treating physicians knew who was in which group. After the twelve weeks, significantly more people who had received 600 mg olamkicept showed a clinical response (improvement in symptoms or endoscopic findings). Symptoms disappeared completely in around 21 percent (compared to 0 percent in the placebo group) and healing of the intestinal mucosa was observed in almost 35 percent (compared to three percent in the placebo group).
The results were first presented at the renowned international gastroenterology conference Digestive Disease Week in December 2021 and published in the journal JAMA in March 2023. "The fact that the international scientific community has now recognized the study results in the peer review process is a major milestone for our research, whose declared goal is to bring results from basic research into clinical application," says Prof. Schreiber. The development of olamkicept as a medicinal product is being carried out by the pharmaceutical company Ferring as licensee together with the Kiel-based biotechnology company CONARIS Research Institute AG and the Chinese pharmaceutical company I-Mab Biopharma.
Original publication:
Shenghong Zhang, Baili Chen, …, Stefan Schreiber & Minhu Chen: Effect of Induction Therapy With Olamkicept vs Placebo on Clinical Response in Patients With Active Ulcerative Colitis - A Randomized Clinical Trial; JAMA. 2023;329(9):725-734. doi:10.1001/jama.2023.1084
More news about the topic:
Olamkicept captures the complex consisting of IL-6 and its soluble receptor in the blood, thereby selectively blocking the trans-signaling pathway, without disturbing the positive effects of IL-6 via the "classic" pathway.
Professor Stefan Rose-John, member of the Cluster of Excellence "Precision Medicine in Chronic Inflammation", former Executive Director of the Institute of Biochemistry at Kiel University (CAU), and head of the Collaborative Research Centre (CRC) 877 "Proteolysis as a Regulatory Event in Pathophysiology".
About the Cluster of Excellence PMI
The Cluster of Excellence "Precision Medicine in Chronic Inflammation" (PMI) is being funded from 2019 to 2025 through the German Excellence Strategy (ExStra). It succeeds the "Inflammation at Interfaces” Cluster, which was already funded in two periods of the Excellence Initiative (2007-2018). Around 300 members from eight institutions at four locations are involved: Kiel (Kiel University, University Medical Center Schleswig-Holstein (UKSH), Muthesius University of Fine Arts and Design, Kiel Institute for the World Economy (IfW), Leibniz Institute for Science and Mathematics Education (IPN)), Lübeck (University of Lübeck, University Medical Center Schleswig-Holstein (UKSH)), Plön (Max Planck Institute for Evolutionary Biology) and Borstel (Research Center Borstel - Leibniz Lung Center).
The goal is to translate interdisciplinary research findings on chronic inflammatory diseases of barrier organs to healthcare more intensively, as well as to fulfil previously unsatisfied needs of the patients. Three points are important in the context of successful treatment, and are therefore at the heart of PMI research: the early detection of chronic inflammatory diseases, the prediction of disease progression and complications, and the prediction of individual responses to treatment.
Press office
fbuhse@uv.uni-kiel.de+49 (0)431/880 4682 https://precisionmedicine.de
Cluster of Excellence "Precision Medicine in Chronic Inflammation"
Scientific Office
Head: Dr. habil. Susanne Holstein Postal
Christian-Albrechts-Platz 4, 24118 Kiel, Germany
Contact: Sonja Petermann
+49 (0)431 880-4850, fax: +49 (0)431 880-4894
spetermann@uv.uni-kiel.de
Twitter: PMI @medinflame