Science publication: key protein relevant for viral infection and hereditary disease discovered
Professor Sabrina Jabs from the Cluster of Excellence "Precision Medicine in Chronic Inflammation" (PMI) investigated the function of a new disease gene with international cooperation.
The starting point of the research was the search for host factors, that are necessary for RNA viruses such as SARS-CoV-2 to replicate. For this purpose, genome-wide CRISPR/Cas knock-out screens in human cell cultures were used to investigate which cells survive after infection with certain viruses. "In other words, in the cell culture, we switched off virtually every gene in the genome at least once and then analysed which gene knock-out causes the cells to survive despite viral infection, because the viruses cannot replicate," explains Professor Sabrina Jabs from the Institute of Clinical Molecular Biology (IKMB) at Kiel University (CAU) and the University Medical Center Schleswig-Holstein (UKSH), Campus Kiel. In this way, Jabs, together with working groups from the Stanford University School of Medicine, USA, as well as the University Medical Center Hamburg-Eppendorf (UKE), not only found important host factors for viral infections, but also discovered a protein – called LYSET – whose function was previously unknown. It is crucial for the proper functioning of the lysosomes and enables insights into the development of a rare lysosomal storage disorder. The study has now been published in the renowned scientific journal Science, together with another study that has found the same gene on the basis of a different research approach. "About a year ago, we learned about the other study led by the German Cancer Research Center (DKFZ) in Heidelberg, exchanged data and decided to submit the work to Science together, rather than racing to be the first to publish the discovery in competition with each other. The publications complement each other, so coordinated publication made sense," said Jabs, who is also a co-author of the second publication.
LYSET is essential for transporting soluble enzymes to lysosomes
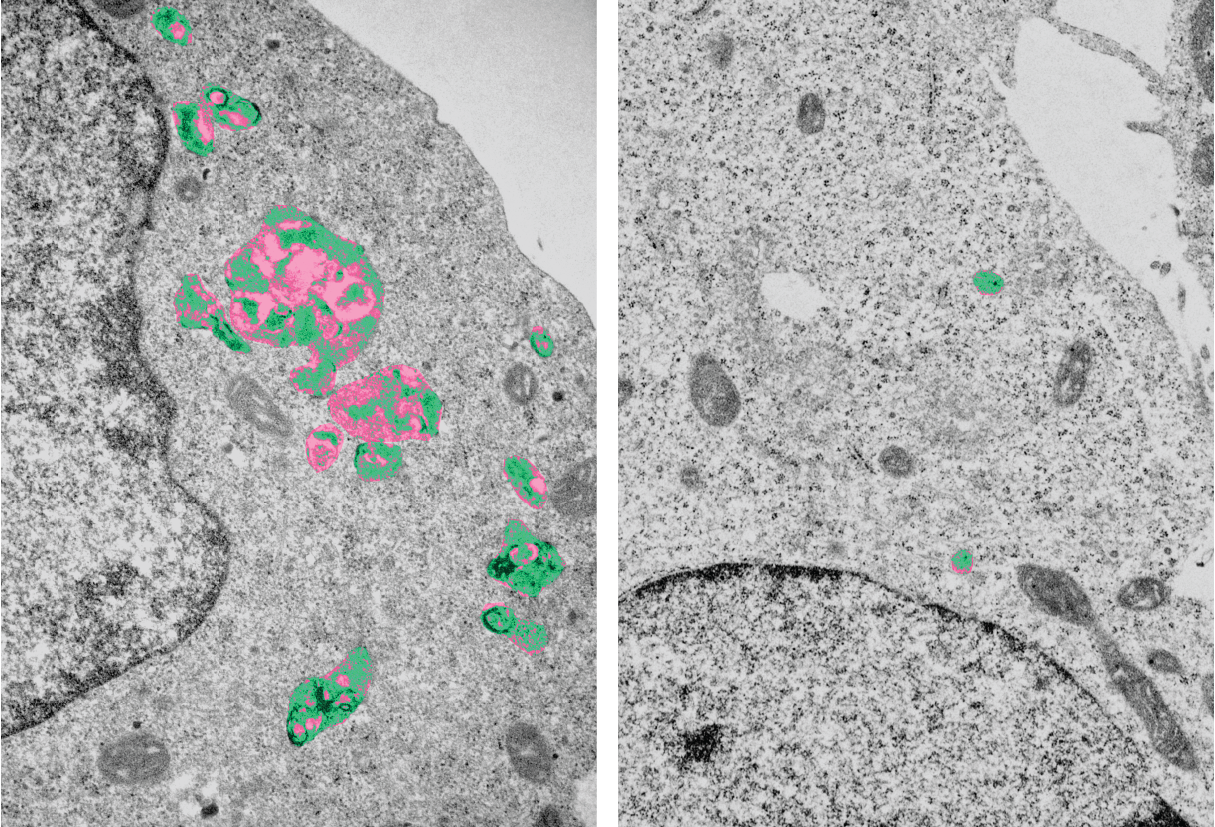
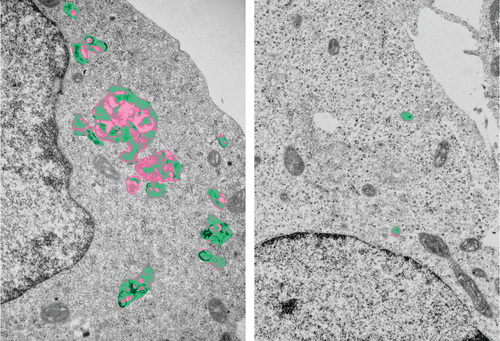
Lysosomes are important degradation locations within cells. They contain a multitude of enzymes for "digesting" non-cellular as well as own cellular material. A malfunction causes (storage) materials to accumulate within the cell. This is the cause of serious clinical damage in patients with lysosomal storage disorders, a group of hereditary metabolic diseases. "Our work identified LYSET as an essential regulator for a fundamentally important transport mechanism within the cell. Mutations in the LYSET gene are associated with a rare lysosomal storage disorder," said Jabs. In cells which lack LYSET, the transport of enzymes to the lysosomes is severely disrupted. In addition to the loss of function of the lysosomes, the knock-out of LYSET also prevents certain viruses from causing damage within the cell. The discovery of this new component of a transport mechanism that is important for cell biology provides fundamental insights into cell physiology, which are relevant for human hereditary diseases and at the same time also for viral infections. In the second Science publication by working groups from Heidelberg and Vienna, the significance of LYSET for the growth of tumour cells was also demonstrated.
Starting point for further research
Since the LYSET protein had not been previously characterised, a series of studies will be conducted in the future in order to further investigate its functions. For example, one question is whether a mutation in the LYSET gene is actually the cause of mucolipidosis (ML), a serious hereditary metabolic disease. The disease gene is unknown in around 10 percent of children who are clinically diagnosed with mucolipidosis. According to Jabs, "We could now analyse biosamples from these patients in order to determine whether there is a mutation in the LYSET gene. We are also interested in the structural characteristics of LYSET which may help us to better understand how it works. The temporary inhibition of LYSET and the lysosomal enzyme transport mechanism could be used as a therapeutic approach to suppress the growth of certain tumours and prevent viral infections."
About Sabrina Jabs
Since 2020, Sabrina Jabs has been a junior professor of functional genomics and single cell analysis at the CAU’s Faculty of Medicine, member of the Cluster of Excellence "Precision Medicine in Chronic Inflammation" (PMI) and Schleswig-Holstein Excellence Chair junior research group leader at the IKMB. She was previously a research associate at the Institut Pasteur in Paris. After studying biochemistry in Leipzig, Hamburg and Lyon, she obtained her doctorate in 2012 at the Freie Universität Berlin, the Max Delbrück Center for Molecular Medicine and the Leibniz Forschungsinstitut für Molekulare Pharmakologie (FMP). Her research within the Cluster of Excellence PMI focuses on host-microbe interactions and how these lead to inflammatory diseases. The cell biologist is particularly active in the relatively new field of epitranscriptomics. This means that she investigates modifications at the level of the messenger RNA molecules, which are responsible for the transmission of genetic information.
Original Publications
- Christopher M. Richards*, Sabrina Jabs*, Wenjie Qiao* et al. The human disease gene LYSET is essential for lysosomal enzyme transport and viral infection. Science (2022). Published online first on September 8, 2022.
DOI: 10.1126/science.abn5648
* These authors contributed equally to the publication. - Catarina Pechincha, et al. Lysosomal enzyme trafficking factor LYSET enables nutritional usage of extracellular proteins. Science (2022). Published online first on September 8, 2022.
DOI: 10.1126/science.abn5637
Scientific contact
Prof. Sabrina Jabs
Institute of Clinical Molecular Biology, CAU and UKSH
+49 431 500-15102
s.jabs@ikmb.uni-kiel.de
Lysosomes (pseudo-coloured) in electron microscopy. Cells lacking LYSET (left image) have enlarged lysosomes with undigested storage material.
About the Cluster of Excellence PMI
The Cluster of Excellence "Precision Medicine in Chronic Inflammation" (PMI) is being funded from 2019 to 2025 through the German Excellence Strategy (ExStra). It succeeds the "Inflammation at Interfaces” Cluster, which was already funded in two periods of the Excellence Initiative (2007-2018). Around 300 members from eight institutions at four locations are involved: Kiel (Kiel University, University Medical Center Schleswig-Holstein (UKSH), Muthesius University of Fine Arts and Design, Kiel Institute for the World Economy (IfW), Leibniz Institute for Science and Mathematics Education (IPN)), Lübeck (University of Lübeck, University Medical Center Schleswig-Holstein (UKSH)), Plön (Max Planck Institute for Evolutionary Biology) and Borstel (Research Center Borstel - Leibniz Lung Center).
The goal is to translate interdisciplinary research findings on chronic inflammatory diseases of barrier organs to healthcare more intensively, as well as to fulfil previously unsatisfied needs of the patients. Three points are important in the context of successful treatment, and are therefore at the heart of PMI research: the early detection of chronic inflammatory diseases, the prediction of disease progression and complications, and the prediction of individual responses to treatment.
Press contact
kerstin.nees@hamburg.de+49 431 880-4682
Cluster of Excellence "Precision Medicine in Chronic Inflammation"
Scientific Office
Head: Dr. habil. Susanne Holstein Postal
Christian-Albrechts-Platz 4, 24118 Kiel, Germany
Contact: Sonja Petermann
+49 (0)431 880-4850, fax: +49 (0)431 880-4894
spetermann@uv.uni-kiel.de
Twitter: PMI @medinflame