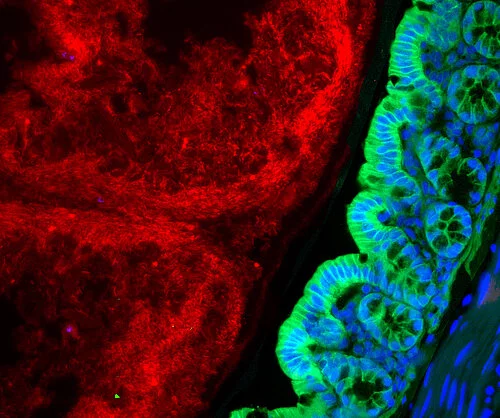
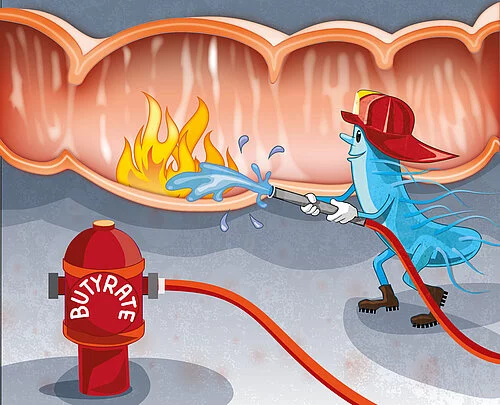
Link between intestinal inflammation and microbiome
Study by the Cluster of Excellence "Precision Medicine in Chronic Inflammation" clarifies the anti-inflammatory effect of the fatty acid butyrate that is formed by intestinal bacteria.
Around 500 to 1,000 different types of bacteria, fungi and other microorganisms colonize our intestines. All of them together form the intestinal microbiome. As we now know, these microbes play an important role in maintaining health. This is especially evident when the composition of the microbiome becomes unbalanced, as is the case in people with chronic inflammatory bowel diseases. Their intestinal microbiome contains fewer types of bacteria than that of healthy people. But so far, precisely how a modified microbiome contributes to the development of diseases is largely unknown. PD Dr. Felix Sommer and his team from the Cluster of Excellence "Precision Medicine in Chronic Inflammation" (PMI) are trying to better understand the complex interactions between the host and their microbiome. While doing so, they encountered an enzyme called hexokinase 2 (HK2). The HK2 enzyme is produced in greater quantities in the event of inflammation and is regulated by the microbiome. "We were able to show in the animal model that by administering the short-chain fatty acid butyrate, the HK2 levels in intestinal epithelial cells were lowered and inflammation was ameliorated. The protective effect of the fatty acid butyrate produced by bacteria was absent in animals where the HK2 enzyme was removed," explained Sommer, head of the Functional Host-Microbiome Research working group at the Institute of Clinical Molecular Biology (IKMB) at Kiel University (CAU) and the University Medical Center Schleswig-Holstein (UKSH), Campus Kiel. The results of the study* have been published in the renowned scientific journal Cell Metabolism.
Enzyme hexokinase 2: high levels in intestinal cells indicate unhealthy state
In cooperation with scientists from Kiel, Lübeck, Munich and Hanover, as well as from the USA and Sweden, Sommer and his team investigated how intestinal bacteria regulate the HK2 enzyme and influence inflammations, including through studies using mice without this enzyme in their intestinal mucosa cells. "These mice were less susceptible to an experimentally-induced intestinal inflammation," reported the first authors Finn Hinrichsen and Jacob Hamm, both doctoral researchers at the IKMB. The experiments also identified microbial factors that regulate the production of HK2: short-chain fatty acids such as acetate and butyrate. These fatty acids are mainly not absorbed through food, they are produced by bacteria in the intestine - but only by very specific intestinal bacteria. “Acetate increased the concentration of HK2 in the intestinal cells, while butyrate decreased the HK2 levels," said Sommer. Regarding the inflammation, he noted: "Administering acetate aggravated an experimental intestinal inflammation in wild-type mice, whereas butyrate had a protective effect. These effects were not present in HK2-deficient mice, i.e. animals that lacked the HK2 enzyme."
Potential target molecule for new anti-inflammatory treatments
The fact that the short-chain fatty acid butyrate, also called butyric acid, stabilizes the intestinal barrier and has an anti-inflammatory effect, was already known from many previous studies. However, the very unpleasant smell of butyrate and its strong laxative effect are obstacles to therapeutic use, for example in cases of chronic inflammatory bowel diseases. "By identifying HK2 as a target of butyrate, we have found a potential entry point for new anti-inflammatory medications. Inhibiting HK2 would be more specific than treatment with butyrate," emphasized Sommer.
Theoretically, it would also be possible to build up the intestinal microbiome by administering fatty acid-producing bacteria, as pointed out by the last author, Professor Philip Rosenstiel. "To date, many approaches here have been rather broad-based. The study provides an important indication that we may actually be able to specifically select individual bacteria in order to control certain metabolic processes in the intestinal mucosa, and thus also inflammation," explained IKMB Director Rosenstiel.
* The study was funded by the Cluster of Excellence PMI, the Research Group FOR 5042 "miTarget: The microbiome as a therapeutic target in inflammatory bowel diseases", the Research Training Group RTG 1743 "Genes, Environment, Inflammation" and the Collaborative Research Centre (CRC) 1182 "Origin and Function of Metaorganisms".
Original publication:
Hinrichsen F, Hamm J, Westermann M, … Rosenstiel P, Sommer F. Microbial regulation of hexokinase 2 links mitochondrial metabolism and cell death in colitis. Cell Metabolism (2021) https://doi.org/10.1016/j.cmet.2021.11.004
Scientific Contact:
Finn Hinrichsen
Institute of Clinical Molecular Biology (IKMB),
CAU, UKSH
+49 (0)431 500-15146
f.hinrichsen@ikmb.uni-kiel.de
Jacob Hamm
Institute of Clinical Molecular Biology (IKMB),
CAU, UKSH
+49 (0)431 500-15146
j.hamm@ikmb.uni-kiel.de
Prof. Dr. Philip Rosenstiel,
Institute of Clinical Molecular Biology (IKMB), CAU, UKSH
+49 (0)431 500-15111
p.rosenstiel@mucosa.de
PD Dr. Felix Sommer,
Institute of Clinical Molecular Biology (IKMB), CAU, UKSH
+49 (0)431 500-15146
f.sommer@ikmb.uni-kiel.de
About the Cluster of Excellence PMI
The Cluster of Excellence "Precision Medicine in Chronic Inflammation" (PMI) is being funded from 2019 to 2025 through the German Excellence Strategy (ExStra). It succeeds the "Inflammation at Interfaces” Cluster, which was already funded in two periods of the Excellence Initiative (2007-2018). Around 300 members from eight institutions at four locations are involved: Kiel (Kiel University, University Medical Center Schleswig-Holstein (UKSH), Muthesius University of Fine Arts and Design, Kiel Institute for the World Economy (IfW), Leibniz Institute for Science and Mathematics Education (IPN)), Lübeck (University of Lübeck, University Medical Center Schleswig-Holstein (UKSH)), Plön (Max Planck Institute for Evolutionary Biology) and Borstel (Research Center Borstel - Leibniz Lung Center).
The goal is to translate interdisciplinary research findings on chronic inflammatory diseases of barrier organs to healthcare more intensively, as well as to fulfil previously unsatisfied needs of the patients. Three points are important in the context of successful treatment, and are therefore at the heart of PMI research: the early detection of chronic inflammatory diseases, the prediction of disease progression and complications, and the prediction of individual responses to treatment.
Press contact
kerstin.nees@hamburg.de+49 431 880-4682
Cluster of Excellence "Precision Medicine in Chronic Inflammation"
Scientific Office
Head: Dr. habil. Susanne Holstein Postal
Christian-Albrechts-Platz 4, 24118 Kiel, Germany
Contact: Sonja Petermann
+49 (0)431 880-4850, fax: +49 (0)431 880-4894
spetermann@uv.uni-kiel.de
Twitter: PMI @medinflame