Agingbrain:partoftheinnateimmunesystemregulatestheblood-brainbarrier
German-Americanresearchteamdemonstratestheroleofthecomplementsystemintheagingprocessofthebrain.
 
Asapersonbecomesolder,manystructuresandfunctionsinthebrainchange.Animportantage-relatedchangeistheincreasedpermeabilityoftheblood-brainbarrier,whichnormallyonlyallowscertainsubstancesfromthebloodstreamintoandoutofthebrain.Amongotherthings,thismayfavorthedevelopmentofneurodegenerativediseasessuchasAlzheimer'sdementia.Aseriesofpreviousstudiessuggeststhatthereducedfunctionoftheblood-brainbarrierislinkedtoimmunologically-relatedinflammatoryprocesseshowever,theexactmechanismsbehindthisarestillhardlyunderstood.Inlaboratorytests,ProfessorJrgKhl,boardmemberoftheClusterofExcellence"PrecisionMedicineinChronicInflammation"(PMI)anddirectoroftheKielInstituteforSystemicInflammationResearch(ISEF),UniversityofLbeck(UzL)andUniversityMedicalCenterSchleswig-Holstein(UKSH),CampusLbeck,togetherwiththeworkinggroupledbyProfessorHuiZheng(HuffingtonCenteronAging,Houston,USA),havenowidentifiedamechanismbywhichtheso-calledcomplementsystem,aspecificpartoftheimmunesystem,regulatesthevascularinflammationandassociateddisturbedfunctionoftheblood-brainbarrierduringtheagingprocess.Theresearchersrecentlypublishedtheirfindingsintherenownedscientificjournal"JournalofClinicalInvestigation.
Thecomplementsystemispartoftheinnateimmunesystem.Itconsistsofmorethan40proteins,whicharepresentinthebloodandasreceptorsonthesurfaceofcells.Theseproteinsincludetheso-calledcomplementfactorsandtheircleavageproducts(proteinfragments),aswellasreceptorsthatrecognizethesefragments.Thesystemplaysanimportantroleinthedefenseagainstmicroorganisms.Itsignalsthepresenceofpathogensaswellasthebindingofantibodiestopathogens,butalsotothebody'sownstructures.However,faultyregulationofthecomplementsystemmayresultinanexcessiveinflammatoryresponseinthecourseofcertaindiseases,whichcausestissuedamage.Whilemostofthecomplementproteinsareproducedintheliver,ithaslongbeenknownthatbothcomplementfactorsaswellascomplementreceptorsarealsoindependentlyproduceddirectlyinthebrain.OfparticularimportanceisthecomplementfactorC3,whichcanbefragmentedintotwoparts,C3bandC3a.C3atriggersitsbiologicaleffectbybindingwiththeC3areceptor,whichispresentoncellssuchasthevascularendothelialcells,i.e.thecellswhichformtheinnerliningofbloodvessels,aswellasvariousbraincells.
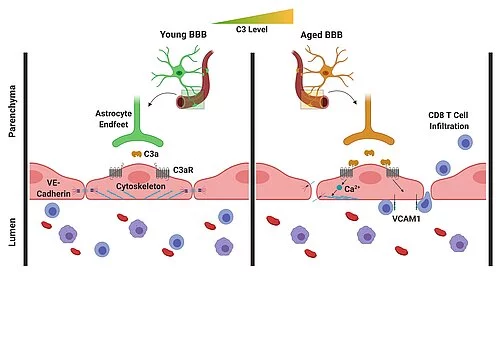
Intherecently-publishedstudy,theresearcherswereabletoshowthatC3/C3aproductionincreasesintheagingbrain,andthereisactivationoftheC3areceptoronvascularendothelialcellsofthebrain.Inresponse,thesecellsincreasinglyproduceacertainadhesionmolecule,leadingtoanincreasedmigrationoflymphocytes(specialimmunecells)intothebrain.Inaddition,theactivationoftheC3areceptoronthevascularendothelialcellsinducesthereleaseofintracellularcalciumions.Thisleadstoadisruptionofaspecificproteinonthevascularendothelialcells,whichisofprimaryimportanceforthebindingofthesecells.Breakingthesebondscausesincreasedpermeabilityoftheblood-brainbarrier.Thismeansthatthebrainisnolongersowellseparatedfromtherestofthebody,sothatinflammationscanalsospreadmoreeasilyfromtheenvironmenttothebrain.
Inordertoinvestigatethisdiseasemechanism,theresearchersdevelopedatransgenicmousemodel,inwhichthemiceweregeneticallyalteredsothattheynolongerproduceC3areceptorsonthevascularendothelialcells.ThespecificeliminationoftheC3areceptorinvascularendothelialcellsnotonlyprotectedthemiceagainstanincreasedpermeabilityoftheblood-brainbarrierinoldage,italsoreducedtheinflammatoryactivityofspecialimmunecellsinthebrain,themicrogliacells,andincreasedthevolumeofthehippocampusandcerebralcortexofagingmice,incomparisonwithmicewiththeC3areceptor.Thehippocampusisofparticularimportanceforlearningandmemoryprocesses.
"Ourfindingsshowanewregulatorymechanismoftheblood-brainbarrierwhichiscontrolledbythecomplementsystem,andtheassociatedimmunecellactivation,inflammationanddegenerationintheagingbrain,"saidKhl."Thedatasuggeststhatthetargetedblockingofindividualelementsofthecomplementsystemcouldleadtoasignificantimprovementinthevascularfunctionandreducethepermeabilityoftheblood-brainbarrier,andtherebyareductioninneuroinflammationandneurodegenerationinoldage,"addedKhl.
Theobservationscouldalsobeinterestingforacuteinflammatoryconditionssuchasstrokesandtraumaticbraininjuriesorneurodegenerativediseases,inparticularAlzheimer's,forwhichageisthebiggestriskfactor."Itisknownfrompreviousstudiesthatthecomplementsystemisalsohighlyactivatedinthesediseases,"explainedKhl. "Thus,ourfindingscouldinfuturealsoenablenewtherapeuticapproachesfortheseage-relateddiseasesofthebrain."
Scientificcontact:
Prof.JrgKhl
nstituteforSystemicInflammationResearch,
UniversityofLbeckandUniversityMedicalCenterSchleswig-Holstein(UKSH),CampusKiel
+49-451-500-51400
Joerg.Koehl@uksh.de
ProfessorJ��rgK��hl,steeringcommitteememberoftheClusterofExcellence"PrecisionMedicineinChronicInflammation"(PMI)andDirectoroftheInstituteofSystemicInflammationResearch(ISEF),UniversityofL��beck(UzL)andUniversityMedicalCenterSchleswig-Holstein(UKSH),CampusL��beck.
Intheagingbrain,C3/C3aproductionincreasesandtheC3areceptorsonvascularendothelialcellsofthebrainareactivated.Inresponse,thesecellsproduceincreasedamountsoftheadhesionmoleculeVCAM-1,whichleadstoanincreasedmigrationoflymphocytesintothebrain.Inaddition,theactivationoftheC3areceptorleadstoamobilizationofcalciumionsinthecells,resultinginadisruptionofthevascularendothelialcadherin,whichisofcentralimportancefortheinterconnectionofthesecells.Breakingtheseconnectionsresultsinanincreasedpermeabilityoftheblood-brainbarrier.
Originalpublication:
PropsonN.E.,RoyE.R.LitvinchukA.,KhlJ.,ZhengH.:EndothelialC3areceptormediatesvascularinflammationandBBBpermeabilityduringaging.J.Clin.Invest.(2020);firstpublishedSep29,2020.DOI:10.1172/JCI140966
AbouttheClusterofExcellencePMI
TheClusterofExcellence"PrecisionMedicineinChronicInflammation"(PMI)isbeingfundedfrom2019to2025throughtheGermanExcellenceStrategy(ExStra).Itsucceedsthe"InflammationatInterfacesCluster,whichwasalreadyfundedintwoperiodsoftheExcellenceInitiative(2007-2018).Around300membersfromeightinstitutionsatfourlocationsareinvolved:Kiel(KielUniversity,UniversityMedicalCenterSchleswig-Holstein(UKSH),MuthesiusUniversityofFineArtsandDesign,KielInstitutefortheWorldEconomy(IfW),LeibnizInstituteforScienceandMathematicsEducation(IPN)),Lbeck(UniversityofLbeck,UniversityMedicalCenterSchleswig-Holstein(UKSH)),Pln(MaxPlanckInstituteforEvolutionaryBiology)andBorstel(ResearchCenterBorstel-LeibnizLungCenter).
Thegoalistotranslateinterdisciplinaryresearchfindingsonchronicinflammatorydiseasesofbarrierorganstohealthcaremoreintensively,aswellastofulfilpreviouslyunsatisfiedneedsofthepatients.Threepointsareimportantinthecontextofsuccessfultreatment,andarethereforeattheheartofPMIresearch:theearlydetectionofchronicinflammatorydiseases,thepredictionofdiseaseprogressionandcomplications,andthepredictionofindividualresponsestotreatment.
Pressoffice
fbuhse@uv.uni-kiel.de+49(0)431/8804682https://precisionmedicine.de
ClusterofExcellence "PrecisionMedicineinChronicInflammation"
ScientificOffice
Head:Dr.habil.SusanneHolsteinPostal
Christian-Albrechts-Platz4,24118Kiel,Germany
Contact:SonjaPetermann
 +49(0)431880-4850,fax:+49(0)431880-4894
spetermann@uv.uni-kiel.de
Twitter:PMI@medinflame